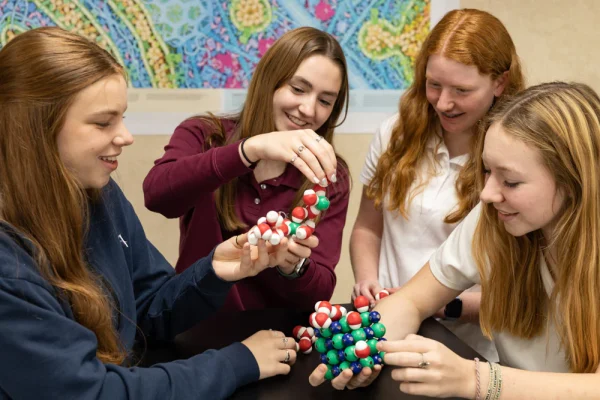Polarity is unforgettable after modeling with these engaging, magnetic molecules. Students love figuring out molecular attractions and repulsions, providing a springboard to more complex chemistry concepts.
Description
Considered the gateway kit for students to develop model literacy, The Water and Salt crystal molecule set is a great beginning-of-the-year activity. The set includes rigorous and engaging materials that provoke, inspire, and invite all students to the learning.
Polarity is unforgettable after modeling with these engaging, magnetic molecules. Students love figuring out molecular attractions and repulsions, providing a springboard to more complex chemistry concepts.
Investigate the polarity of water molecules
Explore ionic, covalent, and hydrogen bonds
Experiment with adhesion, cohesion, and capillary action
Identify and model states of matter
Demonstrate changes in volume between liquid and solid water
Build different types of ice
Simulate solubility with sodium and chloride ions
Build ethane and ethanol to observe interactions with water
Set includes 12 H2O cups and 3 4x4x4 NACL Latice models
Water Kit resources
Water Kit© Next Generation Science Standards
Basic Lessons
More
Water Kit© Contents Per Cup
24 Oxygen* Pieces (Used to Assemble 12 Water Molecule Models)
24 Hydrogen* Pieces (Used to Assemble 12 Water Molecule Models)
1 Chloride* (Chlorine) Model
1 Sodium* Model
2 Carbon Pieces (Used to Assemble 1 Ethane Model)
6 Hydrogen** Pieces without Magnets (Used to Assemble 1 Ethane Model)
1 Post (Used to Assemble 1 Ethane Model)
1 Hydroxyl* Group Model (Preassembled)
*North and south poles on embedded magnets simulate the partially positive and negative charges of oxygen and hydrogen atoms, and chloride and sodium ions.
**The hydrogen pieces for the ethane model do not include embedded magnets, since ethane is nonpolar.
The colorful magnetic sodium chloride ion models are a great addition to the H2O kits. With these models, students will:
Discover the cubic nature of salt crystals
Explore how the positive sodium and negative chloride ions form electrostatic bonds
Examine the efficient lattice packing of the ions
Investigate high melting temperature, brittleness, and cleavage planes
4x4x4 Lattice Contents
- Sodium Ions 32
- Chloride Ions 32
NaCl Resources
Teacher Guides and Student Handouts
NGSS Connections
Activities
More